SEO Meta Description:
Thomson Model of Atom explained in detail—discover its structure, diagram, key features, limitations, and how it shaped modern atomic theory.
Thomson Model of Atom: Structure, Diagram & Limits
Have you ever imagined what an atom looks like? Today, we picture electrons orbiting a nucleus like planets around the sun. But science didn’t start there. In fact, before Rutherford’s gold foil experiment shook the scientific world, there was a simpler—almost charming—idea about atomic structure. It was called the Thomson Model of Atom, and for a time, it made perfect sense.
The Thomson Model of Atom marked a turning point in atomic theory. It came after the discovery of the electron and before the discovery of the atomic nucleus. While it may seem outdated today, this model laid the groundwork for the development of modern atomic physics.
Let’s explore the structure, diagram, features, and limits of the Thomson Model of Atom in a way that’s easy to understand and hard to forget.
Background of the Thomson Model of Atom
Before diving into the structure, it helps to understand the scientific environment of the late 19th century.
John Dalton had already introduced his atomic theory, stating that atoms were indivisible particles. However, things changed dramatically in 1897 when J.J. Thomson discovered the electron through his cathode ray experiment.
This discovery proved that atoms were divisible and contained smaller subatomic particles. As a result, scientists had to rethink atomic structure. That’s when Thomson proposed his famous atomic model in 1904.
The Thomson Model of Atom is often referred to as the plum pudding model. The name might sound funny, but the idea behind it was quite logical for its time.
What Is the Thomson Model of Atom?
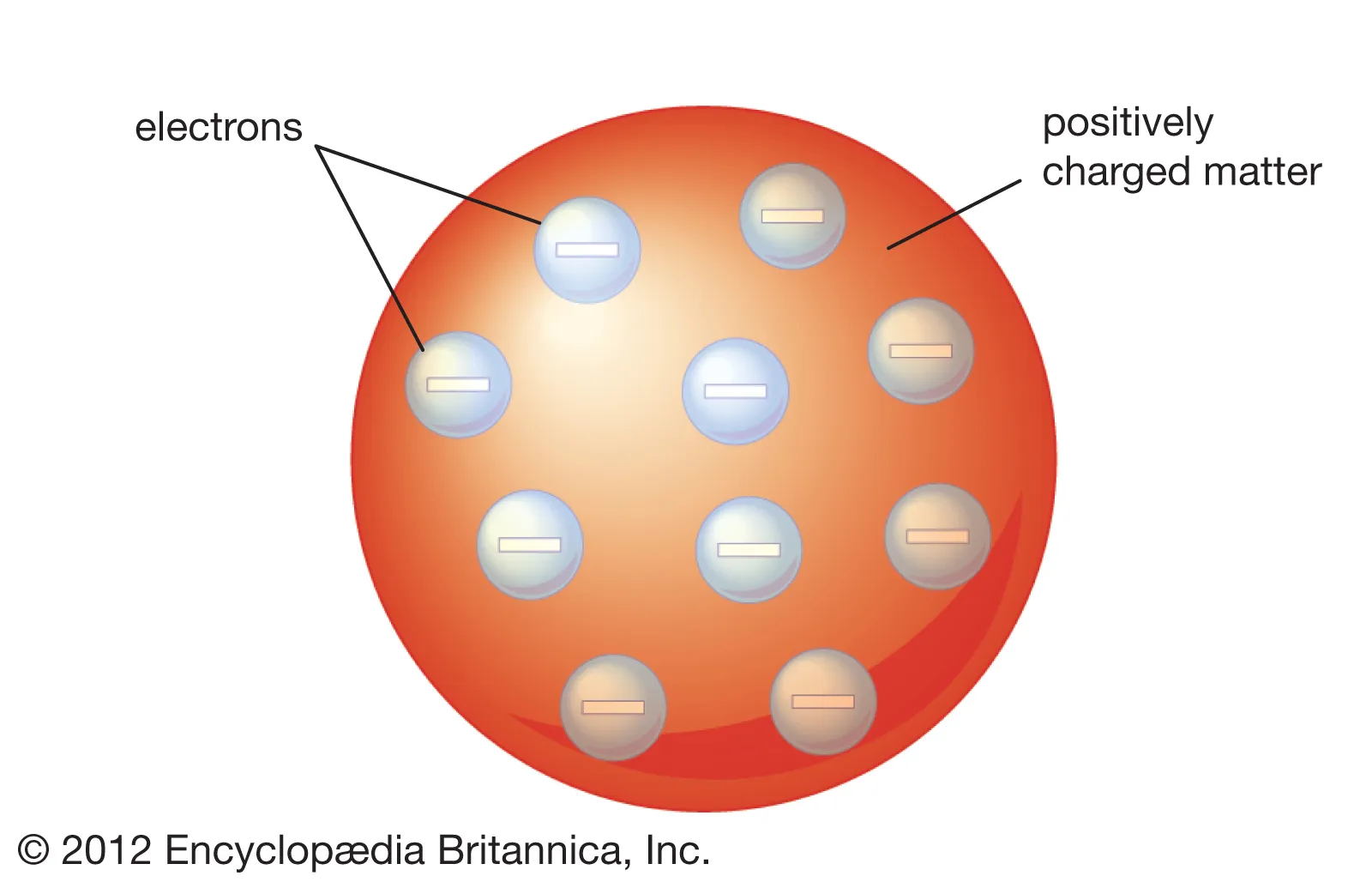
The Thomson Model of Atom describes the atom as a positively charged sphere with negatively charged electrons embedded within it.
Imagine a pudding filled with raisins. The pudding represents the positively charged mass, and the raisins represent electrons scattered throughout it. According to Thomson, the positive charge was evenly distributed across the atom, and electrons were embedded inside to balance the charge.
In simple words:
-
Atom = positively charged sphere
-
Electrons = negatively charged particles embedded in it
-
Overall atom = electrically neutral
This model attempted to explain atomic stability and electrical neutrality using known concepts of charge and mass.
Structure of Thomson Model of Atom
Let’s break down the structure in a clear and organized way.
1. Positively Charged Sphere
According to the Thomson Model of Atom, the atom consists of a uniform distribution of positive charge. This positive charge was not concentrated in a nucleus. Instead, it spread evenly throughout the atom.
This was very different from what we know today.
2. Embedded Electrons
Electrons, discovered by Thomson, were placed inside this positively charged sphere. They were scattered randomly, much like chocolate chips in a cookie.
Key points about electrons in this model:
-
They carried negative charge
-
They were lightweight compared to the positive mass
-
They were evenly distributed
-
Their total negative charge balanced the positive charge
3. Electrical Neutrality
The Thomson Model of Atom explained that atoms are electrically neutral because:
Total positive charge = Total negative charge
This was an important insight and remains true even in modern atomic theory.
Diagram of Thomson Model of Atom
Although we can’t physically draw here, the diagram of the Thomson Model of Atom is simple to visualize.
Picture a circle:
-
The circle represents the positively charged sphere.
-
Small dots inside the circle represent electrons.
If you were to draw it step-by-step:
-
Draw a large circle.
-
Fill the circle with several small negative symbols (–) scattered inside.
-
Label the circle as “Positive charge.”
-
Label the small symbols as “Electrons.”
That’s the classic plum pudding diagram.
For classroom learning, this diagram is often shown to help students understand early atomic models before moving to Rutherford’s nuclear model and Bohr’s model.
Key Features of Thomson Model of Atom
The Thomson Model of Atom had several important features that made it scientifically significant at the time.
Main Features
-
Atom is a uniform sphere of positive charge
-
Electrons are embedded within the sphere
-
No nucleus exists
-
Positive charge is evenly distributed
-
Atom is electrically neutral
Scientific Contributions
The model helped explain:
-
Electrical conductivity
-
Cathode ray behavior
-
Presence of subatomic particles
-
Basic atomic structure
-
Neutral nature of matter
Although simple, the Thomson Model of Atom was the first attempt to describe the internal structure of an atom after electron discovery.
Importance in the History of Atomic Theory
You might wonder: if the model was wrong, why does it matter?
It matters because science evolves step by step. The Thomson Model of Atom played a critical role in shaping future discoveries.
Here’s why it was important:
-
It introduced the idea that atoms contain electrons
-
It challenged Dalton’s indivisible atom concept
-
It opened doors for nuclear physics
-
It inspired further experiments
-
It advanced understanding of electric charge
Without this model, Rutherford may not have designed his famous gold foil experiment.
Limitations of Thomson Model of Atom
Now comes the turning point. While the Thomson Model of Atom was revolutionary, it couldn’t explain certain experimental observations.
1. Failure in Gold Foil Experiment
In 1911, Ernest Rutherford conducted the gold foil experiment. He bombarded thin gold foil with alpha particles.
If the Thomson Model of Atom were correct:
-
Alpha particles should pass straight through with minimal deflection.
However, the results shocked scientists:
-
Most particles passed through
-
Some deflected at large angles
-
A few bounced back completely
This proved that positive charge was not evenly distributed. Instead, it was concentrated in a tiny nucleus.
2. No Explanation for Atomic Stability
The model didn’t explain:
-
How electrons stayed fixed
-
Why electrons didn’t collapse into the positive sphere
-
Atomic energy levels
-
Spectral lines
3. No Nuclear Concept
The biggest flaw? No nucleus.
Modern atomic structure shows:
-
Dense nucleus
-
Protons and neutrons
-
Electrons in orbitals
The Thomson Model of Atom lacked this core idea.
4. Incomplete Mass Distribution
The model assumed positive mass was uniform. Later discoveries showed:
-
Most atomic mass is in the nucleus
-
Electrons contribute very little mass
Clearly, the structure needed revision.
Comparison with Other Atomic Models
Understanding the Thomson Model of Atom becomes easier when compared with other atomic models.
| Model | Key Idea | Positive Charge | Electrons | Nucleus |
|---|---|---|---|---|
| Dalton Model | Atom is indivisible | Not defined | None | No |
| Thomson Model | Plum pudding structure | Uniform sphere | Embedded | No |
| Rutherford Model | Nuclear model | Concentrated in center | Orbit nucleus | Yes |
| Bohr Model | Quantized orbits | In nucleus | Fixed energy levels | Yes |
This comparison highlights how atomic theory evolved over time.
Advantages of Thomson Model of Atom
Even though it had flaws, the model offered some benefits.
-
First model to include electrons
-
Explained atomic neutrality
-
Simple and easy to understand
-
Provided foundation for future atomic research
-
Advanced study of subatomic particles
In many textbooks, the Thomson Model of Atom is still taught because it represents a key milestone in scientific progress.
Why It Is Called the Plum Pudding Model
The name comes from a British dessert popular during Thomson’s time.
-
Pudding = positively charged sphere
-
Plums or raisins = electrons
It’s a vivid analogy, and honestly, it makes the concept easier to remember.
Even today, students often recall the Thomson Model of Atom using this simple food comparison.
Real-World Relevance of Thomson Model of Atom
You may think this model is outdated. However, its influence still appears in modern physics education.
For example:
-
It introduced subatomic particle concepts
-
It paved the way for quantum mechanics
-
It shaped modern atomic models
-
It contributed to early research in electromagnetism
-
It influenced development of nuclear science
Science builds on past mistakes as much as past successes.
Frequently Asked Questions
Who proposed the Thomson Model of Atom?
The Thomson Model of Atom was proposed by Sir J.J. Thomson in 1904 after discovering the electron.
What was the main idea of the Thomson Model of Atom?
The main idea was that the atom consists of a positively charged sphere with electrons embedded within it.
Why did the Thomson Model fail?
It failed because it could not explain the results of Rutherford’s gold foil experiment or the existence of a nucleus.
Is the Thomson Model still valid today?
No, it has been replaced by more accurate atomic models, but it remains important historically.
Key Terms Related to Thomson Model of Atom
To boost clarity and deeper understanding, here are related atomic theory concepts:
-
Atomic structure
-
Subatomic particles
-
Electron discovery
-
Cathode ray experiment
-
Electric charge
-
Positive charge distribution
-
Negative electrons
-
Atomic neutrality
-
Nuclear model
-
Gold foil experiment
-
Alpha particle scattering
-
Atomic mass
-
Proton discovery
-
Neutron discovery
-
Quantum theory
-
Energy levels
-
Orbital structure
-
Electromagnetic radiation
-
Atomic stability
-
Atomic number
-
Isotopes
-
Electron cloud
-
Valence electrons
-
Ion formation
-
Matter composition
-
Periodic table development
-
Electromagnetic fields
-
Plasma physics
-
Fundamental particles
-
Atomic experiments
-
Physics revolution
-
Scientific model evolution
-
Rutherford scattering
-
Classical physics
-
Modern chemistry
-
Atomic nucleus
-
Charge balance
-
Electron configuration
-
Bohr theory
-
Wave-particle duality
-
Atomic spectra
-
Particle physics
These related ideas help position the Thomson Model of Atom within the broader context of atomic and molecular physics.
Final Thoughts on Thomson Model of Atom
The Thomson Model of Atom may not be scientifically accurate today, but it represents a bold leap forward in understanding matter.
It broke the idea that atoms were indivisible. It introduced electrons into atomic theory. It explained electrical neutrality. Most importantly, it sparked new experiments that led to the discovery of the nucleus and the development of modern atomic models.
Science is rarely perfect on the first try. Instead, it evolves. The Thomson Model of Atom reminds us that even imperfect ideas can push humanity toward greater discoveries.
If you found this explanation helpful, consider sharing it with classmates or leaving a comment with your thoughts. Understanding atomic structure becomes much easier when we explore how each model built upon the last.
And who knows? The next big scientific breakthrough might come from questioning today’s “perfect” models—just like Thomson once did.